Home Articles Govt panel says no to Covovax trial on children aged 2-17 years,...
KEY STORY
-
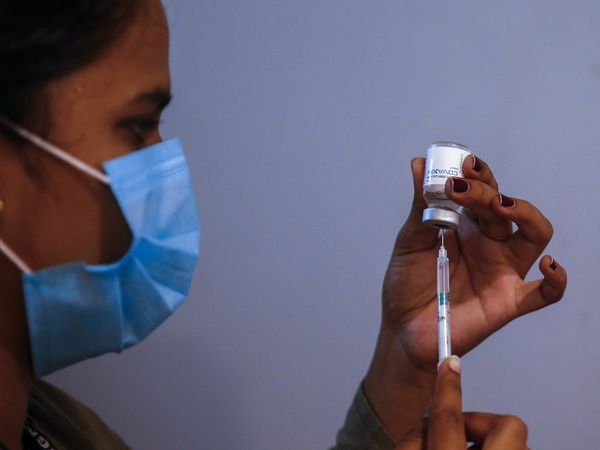
An expert government panel has recommended against granting permission to the Serum Institute of India (SII) to conduct the phase 2/3 trial of COVID-19 vaccine Covovax on children aged two to 17 years.
-
The SII had applied to the Drugs Controller General of India (DCGI) on Monday seeking permission for conducting a trial of Covovax on 920 children, 460 each in the 12-17 and 2-11 age groups, at 10 sites.
-
However, the subject expert on COVID-19 of the Central Drugs Standard Control Organisation (CDSCO) said that the vaccine had not been approved in any country.
SII must submit safety and immunogenicity data of Covovax
-
It further recommended that the Pune-based company should submit the safety and immunogenicity data of Covovax from the ongoing clinical trial in adults for considering the conduct of a clinical trial in children.
-
Sources told PTI that the recommendations are learnt to have been approved by the DCGI.
- US-based vaccine maker Novavax Inc. had announced a licence agreement with the SII for the development and commercialisation of NVX-CoV2373, its COVID-19 vaccine candidate, in low and middle-income countries and India In August 2020.
India to allow entry of Covovax after approval from USFDA
The clinical trials of Covovax began in India in March, and the SII plans to launch it by September for adults.
The Union government expects 200 million doses of Covovax to be available between August and December.
India will be allowing the allow entry of this vaccine once it gets approval from the USFDA.
In a tweet on its official handle, the company said, “A new milestone has been reached; this week, we began our first batch of Covovax at our facility, here in Pune.”
In June, Novavax said it got promising results from its PREVENT-19 phase 3 trials in the US and Mexico, with an overall efficacy of 90.4 per cent.